Exploring the Link Between Stomach Cancer and Blood Type A: A One Health Perspective
One Health Newsletter: Volume 17, Issue 1

Image Credit: Photo by Aleksandr Berdyugin (bermixstudio) on Unsplash. Used under the Unsplash License.
Introduction
tomach cancer, also known as gastric cancer, is the fifth most common cancer in the world, responsible for over one million cases and approximately 769,000 deaths in 2020 (World Health Organization, 2025). Many factors contribute to the development of stomach cancer, such as genetic factors, such as blood type A, as well as non-genetic risk factors like age, sex, ethnicity, and lifestyle (Poorolajal et al., 2020). Some of these factors can be controlled, like lifestyle. Others cannot be controlled, as genetic predisposition (Poorolajal et al., 2020). In One Health, stomach cancer can be analyzed beyond human health alone, considering how environmental exposures, dietary habits, and microbial infections contribute to its incidence. One Health emphasizes the interconnectedness of human, animal, and ecological health. One Health, which integrates human, animal, and environmental health, is crucial in understanding diseases such as stomach cancer, which is influenced by ecological pollutants, zoonotic infections such as Helicobacter pylori infection, dietary choices, and genetic predispositions such as blood type A. By adopting a One Health lens, we can identify more comprehensive strategies for prevention and control.
Environmental and Dietary Risk Factors
Environmental exposures and dietary habits significantly shape the risk of stomach cancer. Consumption of smoked, salted, and pickled foods, which contain high levels of nitrates and nitrites, has been associated with increased risk of gastric malignancies. Conversely, a diet rich in fresh fruits and vegetables, which provide protective antioxidants and fiber, may help reduce this risk (World Cancer Research Fund, 2022). Additionally, alcohol and tobacco use are well-established contributors to gastric cancer. Both substances introduce carcinogens that damage the stomach lining, leading to chronic inflammation and genetic mutations (American Cancer Society, 2021). Excess body weight and gastroesophageal reflux disease (GERD) are also contributing factors. Obesity promotes systemic inflammation and alters gut microbiota, while GERD increases acid exposure to the stomach lining, creating a hostile environment for cellular integrity and increasing cancer risk (National Cancer Institute, 2023).
Furthermore, environmental contaminants, such as heavy metals, nitrates in drinking water, and mycotoxins in food, have been linked to stomach cancer development. These substances can cause cellular damage and disrupt normal gastric functions, reinforcing the need for public health interventions to regulate food and water safety (Wang et al., 2022). One Health addresses interconnected factors ranging from individual lifestyle choices to broader environmental policies and plays a crucial role in reducing stomach cancer incidence.
Microbial and Zoonotic Risk Factors: The role of Helicobacter pylori
One of the most significant risk factors for stomach cancer is infection with Helicobacter pylori (H. pylori), a bacterium that colonizes the stomach lining. H. pylori was found in 22.2% of animal samples by antibody tests and 16% by stool antigen tests, especially in cats (28%) and dogs (24%), and the detection of the gImM gene of H. pylori in both humans and animals–which encodes phosphoglucosamine mutase, an enzyme essential for bacterial cell wall synthesis–serves as a highly specific and reliable molecular marker for H. pylori detection. In the referenced study, PCR assays targeting gImM confirmed H. pylori in 77.8% of antigen-positive human stool samples and 38.4% of milk samples from cattle, buffaloes, and sheep, reinforcing concerns about Zoonotic transmission via animal products and suggesting possible zoonotic transmission (Shaaban et al., 2023). Chronic infection with H. pylori leads to inflammation, DNA damage, and an increased risk of gastric malignancy (Toller et al., 2011). A primary study in Peru showed that children who drank from external water sources had significantly higher rates of H. pylori infection, supporting the hypothesis of waterborne transmission (Klein et al., 1991). Addressing this issue requires coordinated efforts between the veterinary and public health sectors. One Health involves enhancing water quality monitoring, implementing targeted hygiene and sanitation interventions at the community level, and integrating epidemiological surveillance of H. pylori in human and animal populations.
Blood Type A and Its Role in Stomach Cancer
According to Wang et al. (2012), individuals with blood type A have a 1.34 times higher risk of developing gastric cancer compared to other blood types. This increased susceptibility may be linked to immune responses and a higher likelihood of H. pylori infection. H. pylori infection causes chronic inflammation and cellular changes in the gastric lining, which, when combined with genetic predispositions such as blood type A, further elevate cancer risk (Poorolajal et al., 2020).
Studies indicate that H. pylori infection is more prevalent among individuals with blood type A, possibly due to variations in mucosal antigen expression that favor bacterial adhesion. While environmental and lifestyle risk factors such as high salt intake, smoking, and alcohol consumption also contribute to stomach cancer risk (Poorolajal et al., 2020), these affect all blood types. What sets blood type A apart is the compounded risk from both microbial and genetic factors.
Early detection is particularly difficult, as high-risk individuals may be asymptomatic during the initial stages. Wang et al. (2012) emphasized the importance of targeted screening and prevention strategies for blood type A individuals. One Health emphasizes improved sanitation, water safety, and cross-sector collaboration to help mitigate H. pylori-associated gastric cancer risk, particularly in genetically vulnerable populations.
Summary of Reported Risk Factors from Existing Literature
The authors also highlighted that people with blood type A are more susceptible to H. pylori infections. As shown in Table 1, various risk factors contribute to stomach cancer, with H. pylori infection being the most significant, followed by high-salt diets, smoking, and environmental contaminants.
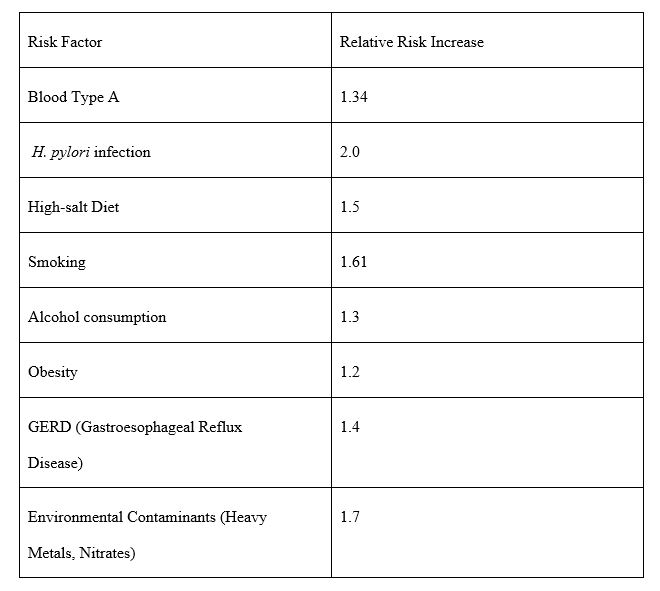
Table 1: Relative Risk Increase for Stomach Cancer Based on Key Risk Factors. Note: Relative risk values indicate the increased likelihood of developing stomach cancer due to various risk factors. Higher values suggest a stronger association with disease. Sources: Adapted from Wang et al. (2022) and Poorolajal et al. (2020). The table was created by the author using data reported in both studies.
Refocusing Public Health Strategies Through a One Health Lens
Although genetic factors such as blood type A are non-modifiable, they interact with modifiable risks central to One Health, such as microbial infections, environmental contamination, and zoonotic exposure. For example, individuals with blood type A are more susceptible to H. pylori, a zoonotic bacterium linked to gastric cancer (Wang et al., 2012; Shaaban et al., 2023). This risk is compounded by poor water sanitation, as demonstrated by higher H. pylori infection rates among children in Peru who relied on external water sources (Klein et al., 1991).
Environmental contaminants like nitrates and heavy metals—often from agricultural runoff, industrial pollution, and poor waste management—also contribute to gastric cancer (Wang et al., 2022). Nitrates, for instance, can contaminate water shared by humans and animals, while heavy metals accumulate in soil and crops, entering the food chain. A Hungarian study found a link between high nitrate levels in drinking water and gastric cancer mortality (Sandor et al., 2001). These examples illustrate how genetic susceptibility, microbial threats, and environmental exposures converge, indicating the need for early screening, improved water safety, and environmental protections. One Health promotes collaboration across disciplines to lessen disease impact and strengthen public health outcomes (National Cancer Institute, 2023).
References
American Cancer Society. (2021). Stomach cancer causes, risk factors, and prevention. Retrieved from https://www.cancer.org/cancer/types/stomach-cancer/causes-risks-prevention/risk-factors.html
Klein, P. D., Graham, D. Y., Gaillour, A., Opekun, A.R., and Smith, E. O. (1991). Water source as a risk factor for Helicobacter pylori infection in Peruvian children. The Lancet, 337(8756), 1503-1506. https://doi.org/10.1016/0140-6736(91)93196-g
National Cancer Institute. (2023). Stomach cancer causes and risk factors. National Institutes of Health. https://www.cancer.gov/types/stomach/causes-risk-factors.
National Day Calendar. (2025). Stomach cancer awareness month |November [Photograph]. © 2025 National Day Calendar. Retrieved from https://www.nationaldaycalendar.com/november/stomach-cancer-awareness-month-november
Poorolajal, J., Moradi, L., Mohammadi, Y., Cheraghi, Z., and Gohari-Ensaf, F. (2020). Risk factors for stomach cancer: A systematic review and meta-analysis. Epidemiology and Health, 42,1-8. https://doi.org/10.4178/epih.e2020004
Sandor, J., Kiss, I., Farkas, O., and Ember, I. (2001). Association between gastric cancer mortality and nitrate content of drinking water: ecological study on small area inequalities. European Journal of Epidemiology, 17(5), 443-447. https://doi.org/10.1023/a:1013765016742
Shaaban, S. I., Talat, D., Khatab, S. A., Nossair, M. A., Ayoub, M. A., Ewida, R. M., and Diab, M. S. (2023). An investigative study on the zoonotic potential of Helicobacter pylori. BMC Veterinary Research, 19, Article 16. https://doi.org/10.1186/s12917-023-03572-w
Toller, I. M., Neelsen, K. J., Steger, M., and Müller, A. (2011). Carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double-strand breaks and a DNA damage response in its host cells. Proceedings of the National Academy of Sciences, 108(36), 14944-14949. https://doi.org/10.1073/pnas.1100959108
Wang. L., Miao, C., He, Y., Li, H., Zhang, S., Li, K., Liu, H., Li, W., Zhao, J., Xu, Y., Tang, H., and Zhao, Q. (2022). The influence of heavy metals on gastric tumorigenesis. Journal of Oncology, 2022(1), e6425133. https://doi.org/10.1155/2022/6425133
Wang, Z., Liu, L., Ji, J., Zhang, J., Yan, M., Zhang, J., Liu, B., Zhu, Z., and Yu, Y. (2012). ABO blood group system and gastric cancer: A case-control study and meta-analysis. International Journal of Molecular Sciences, 13(10), 13308–13321. https://doi.org/10.3390/ijms131013308
World Cancer Research Fund. (2022). Diet and stomach cancer risk. Retrieved from https://www.wcrf.org/preventing-cancer/cancer-types/stomach-cancer
World Health Organization. (2025). Global cancer statistics 2025. Retrieved from https://www.who.int/news-room/fact-sheets/detail/cancer.
More Articles in this Issue