One Health in Action: A Field Pilot Study on Rabies in Ethiopia
Rabies claims the lives of approximately 24,000 people annually in Africa, mainly acquired through bites from unvaccinated domestic dogs (Broban et al. 2018). The lack of a curative treatment for human cases of rabies, coupled with poor access to post-exposure treatment (PET), render traditional healers and holy wells as popular places to be visited by rabies victims in Ethiopia. Hence, the majority of rabies-exposed people (mainly through dog bites) do not receive the expected medical assistance (e.g., PET) in primary health centers after exposure. Moreover, the rapid expansion of urban areas in Ethiopia, coupled with inadequate waste management practices has created an excellent niche for a growing population of free-roaming dogs (Figure 1).

Figure 1. Street Dogs in Yabelo Town of the Borena Zone in Ethiopia (Credit: Jaldessa Galgalo, June 2015).
My passion to study the burden of rabies began in 2012, after learning about the magnitude of underreporting of human rabies deaths in Tanzania (Cleaveland et al. 2002). With respect to the limited reporting health system in Ethiopia and other low or medium income countries, I hypothesized a similar situation in Ethiopia, in collaboration with researchers at the School of Business Economics at Wageningen University (Netherlands). Through extensive fieldwork, the project aimed to explore the health and economic burden of rabies in human and livestock. That data could be used to leverage a One Health approach to disease prevention in Ethiopia, including the implementation of a cost-effective canine vaccination strategy.
Exploring the Burden of Human Rabies
In order to obtain a reasonable data set on the number of rabies exposures and deaths, developing an extensive bite case search was essential. This study was conducted in three selected districts of Ethiopia to account for environmental variations (Bishoftu, Lemuna-bilbilo, Yabelo) (Figure 2). First, Bishoftu district is an urban area located 45km southeast of Addis Ababa, with a relatively robust infrastructure and health centers accessible within a reasonably short distance. Second, Lemuna-bilbilo is a rural district located in the central highlands of Ethiopia, where most people live on a mixed crop and livestock farming system. Third, Yabelo is a rural district in the lowlands of southern Ethiopia, where a majority of the people are pastoralists. Both rural districts have poor road and health facility coverage. Each district has one or more public health centers delivering medical services, including PET for rabies, and every village within a district has a local health extension worker (i.e., primary care center).
Dog bite victims suspected of rabies were traced using data collected at the health centers, and unregistered bite cases were identified by interviewing community members. After tracing, victims and/or their families were contacted and interviewed about their compliance with PET, health impact of the bite, incurred costs, and behavioral manifestation of the biting animal. Based on these data, health burden and cost of rabies were assessed.
Organizing a Visit to the Health Center and Collecting Essential Data: As we visited health centers providing PET, we obtained a list of animal bite victims registered by the districts’ health centers between September 2013 and August 2014. Health extension workers also performed an extensive search for non-registered bite victims by asking the victims whether they knew other people who were bitten but did not visit a health center. In Ethiopia, most neighborhoods are socially close to each other and are well informed about events happening to households in their village (Teshome et al. 2014).
Knocking on the Bite Victim’s Door: We contacted the dog bite victims and interviewed them using a structured questionnaire. In cases where the victim was deceased or a child, an adult family member was interviewed. The questionnaire included the following information: 1) demographic features; 2) affected anatomic extremity; 3) characteristics of the biting dog (behavior and ownership); 4) whether the victim visited a health center and received PET; 5) whether the person survived; and 6) expenditures incurred related to treatment of the bite. The behavioral manifestation and clinical signs of the biting dogs, as described by the victims, were subsequently used to categorize the dog as rabid or non-rabid (Tepsumethanon et al. 2005).
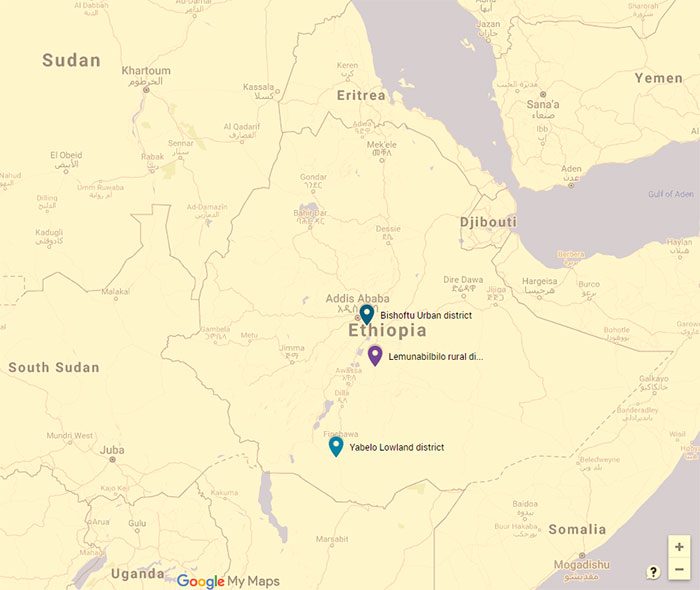
Figure 2. District Study Sites for Rabies, Ethiopia (2013-2014). Legend: The districts of Lemuna-bibilo and Bishoftu have a mixed crop-livestock production system and Yabelo has a pastoral production system. (Source: Google Maps).
Accordingly, we had to systematically identify which exposures were potentially from rabid dogs. The number of dog bite victims who visited health centers (n=450) and those who did not visit the health center (n=182) are represented in Figure 3, including information with regard to actions and outcomes. All bite victims (n=632) were interviewed using a structured questionnaire, according to our six criteria mentioned above. Among them, 360 victims from those who visited health centers and 105 from those who were not bitten by a suspected rabid dog received either sufficient doses of prophylaxis, at least one dose, or no PET at all. Similarly, victims who did not visit a health center either went to traditional healers or did not seek treatment. All deaths (n=12) resulted from suspected rabid dog bites. Figure 3 presents the types of costs considered under each action taken.
|
Ultimately, we were able to estimate annual rabid dog exposures per urban, rural highland, and rural lowland district at 135, 101, and 86 bites, respectively, which led to 1, 4, and 3 deaths per 100,000 inhabitants, respectively. In the same order, average costs per completed PET resulted in USD$23 to $40, which was significantly higher in rural districts. Weighted extrapolation of results to the national level indicated an annual estimate of approximately 3,000 human deaths resulting in about 93,000 DALYs per year and 97,000 exposed persons requiring on average USD$2 million treatment costs per year countrywide. |
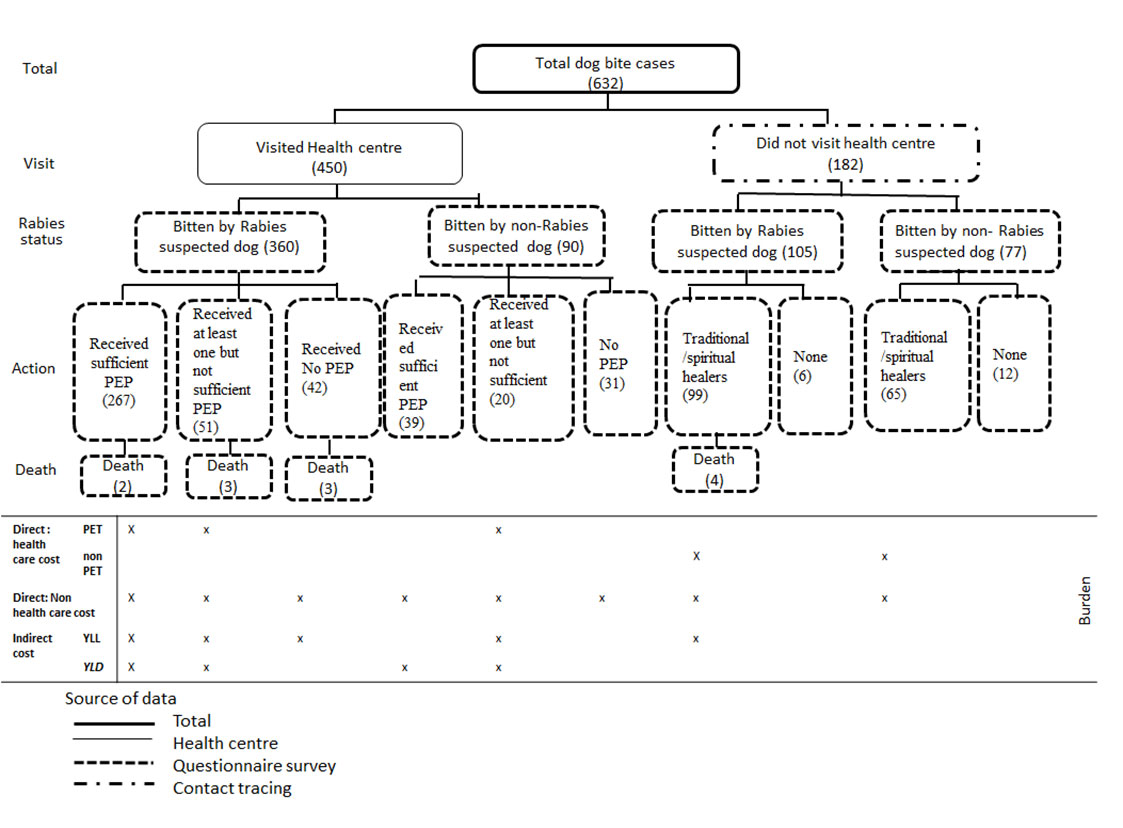
Figure 3. Dog Bite Victim Identified During the Rabies Study (2013-2014) in Ethiopia. The legend shows the classification of total dog bite cases as to their health-seeking behavior. The number of cases per state is in brackets. (Source: Beyene et al. 2018)
Figure 4. Interviewing Individual Rabies Victims in Yabelo District, Southern Ethiopia (left). Dog bite on the left leg (right). (Credit: Jaldessa Galgalo, June 2015)
Understanding the Victim’s Behavior to Seek Treatment Following Rabies Exposure: The questionnaire posed to the victims and/or their families (Figure 4) contained the following sections: (1) demographics and religion of the victim; (2) income; (3) characteristics of the bite (severity and affected extremity); (4) behavioral manifestations, ownership, and fate of the biting animal; (5) distance to the nearest health center that could deliver PET; (6) whether the victim sought medical or traditional treatment; and (7) the number of PET doses received.
|
The overall likelihood of seeking medical services following rabies exposure was higher for people bitten by dogs of unknown ownership. Victims with severe wounds and bites on the leg, and those living close to the nearest health center also sought treatment more frequently. The effect of distance became less important as living in the urban district increased the likelihood of seeking treatment. |
The Extent of Rabies in Cattle: Similar to the health and economic burden of rabies in humans, the purpose of this part of the study was to assess the economic burden of rabies in cattle. Like human cases, livestock rabies is a reportable disease in Ethiopia. However, due to the nature of passive surveillance requiring people to report, authorities rarely receive reports. For this study, we visited administrative zones, Arsi and Borena, which were selected for their representation of different types of subsistence farming systems (Figure 5). Arsi represents a mixed crop-livestock production system and is located in the central highlands about 150km east of Addis Ababa. Borena represents a pastoral production system and is located in the southern lowlands (semi-arid) about 600km south of Addis Ababa.
For Arsi, we visited Munessa and Lemuna-Bilbilo districts, and for Borena, Yabelo, and Dugda Dawa districts. Five villages were then chosen per district based on logistical feasibility. Consequently, 20 villages were selected resulting in a sample size of 532 livestock-owning households, 248 in the mixed crop-livestock system, and 284 in the pastoral system.
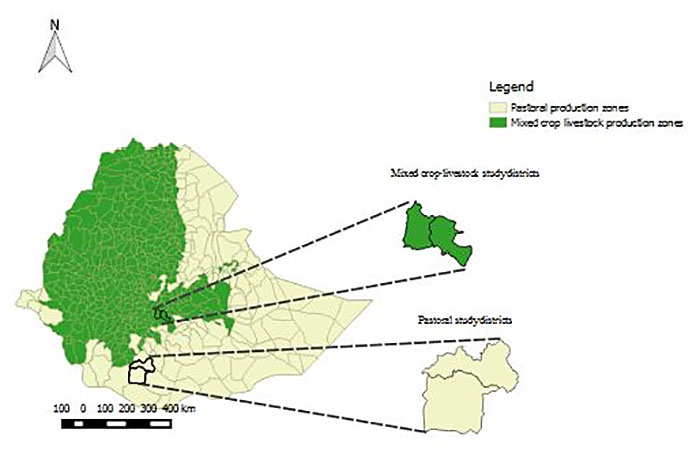
Figure 5. Map of Ethiopia Showing the Mixed Crop-Livestock Production System. The two administrative zones in Arsi are shown in green, and the pastoral production system areas are shown in yellow. The two districts (namely, Munessa and Lemuna-Bilbilo) and two districts in Borena administrative zones (namely, Yabelo and Dugda Dawa) are magnified and highlighted also in green and yellow, respectively.
We performed a preliminary exploration using a focus group to verify the presence of rabies in the selected areas and to determine the ability of livestock owners to identify the disease among their livestock. The preliminary exploration included a group discussion with elder livestock owners in each of the 20 selected villages. This was followed by an individual interview of each livestock-owning household that volunteered to participate in each of the selected villages, using a structured questionnaire to assess the direct economic impact of rabies in the mixed crop-livestock and pastoral systems. Livestock owners included fewer females than males, due to a gender disparity commonly observed for large animal ownership in Ethiopia (Debela, 2017).
Participatory Appraisal: In each of the selected villages, we asked village administrators to identify 15 elder livestock owners who had lived in the village for more than 20 years and were older than 50 years of age.We invited these elders to participate in a group discussion on zoonotic diseases, including 10-12 livestock owners in each of the selected villages (Figure 6). Each group was asked to identify and list the local names of zoonotic diseases that are transmitted to cattle and humans and which have occurred in their area recently during the past five years.
Once the participants had listed the local names of recently occurring zoonotic diseases, they were asked to describe the clinical signs of these diseases in cattle, which they either knew or had observed in their own herds. Subsequently, the group was asked to rank these diseases according to five criteria described elsewhere (Okell et al. 2013) including: 1) perceived risk of the disease in terms of susceptibility and severity to human health; 2) perceived possibility to treat and prevent the disease in cattle; 3) perceived mortality; and 4) morbidity rate in cattle. To identify the English name of each disease, we compared the description of clinical signs given by the participants with the description of a disease in the Merck Veterinary Manual (Kabeta et al. 2015; Kahn et al. 2010).

Figure 6. Participatory Discussion on Rabies in Yabelo, Southern Ethiopia (2013-2014). (Credit: Jaldessa Galgalo, July 2015)
|
Based on the 20 group discussions, a total of nine zoonotic diseases were identified that could spread from animals to humans: Rabies, Anthrax, Brucellosis, Tuberculosis, Teania, Mange mite, Wart, “Liver disease” and “Milk contaminant”. |
Conversation with the Cattle Owners: Once the preliminary exploration indicated the presence of rabies in cattle in a village, each livestock-owning household was interviewed to assess the extent to which the household’s cattle were affected by rabies or bitten by suspected rabid dogs during the past year (September 2013 to August 2014). A structured questionnaire translated into the local language, Oromifa, was administered. In Ethiopia, cattle are generally slaughtered when bitten by a suspected rabid dog, and households were explicitly asked about their experiences with affected and exposed cattle (bitten by a suspected rabid dog). The questionnaire started with a general question about the experience of rabies in the households’ cattle during the last year, followed by specific questions on the cattle that were considered to be affected by rabies and/or bitten by a suspected rabid dog. These questions covered the gender and estimated age of the cattle, clinical symptoms of suspected rabid dogs in cases where cattle were bitten, amount of money received for the ‘sale’ of meat from bitten cattle, and clinical signs observed in cattle.
Cattle Exposed to a Known Suspected Rabid Dog: If the livestock owner had cattle exposed to a known or suspected rabid dog, then the rabies status of the dog was evaluated based on the six criteria for rabies diagnosis in living dogs, as previously described (Tepsumethanon et al. 2005) (Figure 7). If the dog was determined to be rabid, then the bitten cattle were considered exposed to rabies.
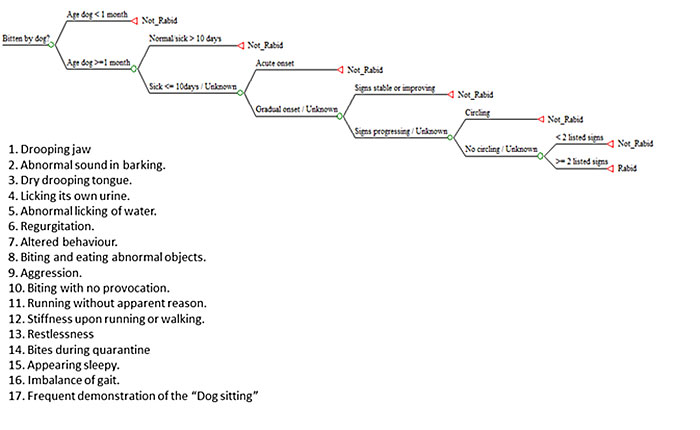
Figure 7. Rabies Status of a Biting Dog (Source: Jibat et al. 2016). Legend: The decision tree algorithm is based on the six criteria previously described (Tepsumethanon et al. 2005) to retrospectively assess the rabies status of a biting dog. The final criterion in the decision tree refers to the list of 17 clinical signs listed.
Cattle Showing Clinical Signs of Rabies without Known Exposure: If livestock owners indicated their cattle exhibited neurological signs without knowing whether the cattle were exposed to a suspected rabid dog, then the evaluation of the rabies status of the cattle was done by comparing the clinical signs described by the livestock owner with the clinical signs of rabies as described in scientific literature (Kahn et al. 2010). To verify the cattle owner's statements, we asked the neighbors if they had seen or heard that the cattle were sick from rabies or bitten by a suspected rabid dog. As cattle are economically important, cattle losses due to disease or any other cause are well known in the neighborhood. We evaluated that one in five livestock-raising households lose cattle due to an attack from a rabid dog, jeopardizing livelihoods and food security for affected households.
|
The total population of households in the selected villages participated in the survey, as every household had at least one head of cattle and no households refused to participate in the survey. Of the 248 herds surveyed in the mixed crop-livestock system, 52 experienced rabies in cattle, with a total of 72 affected cattle. In the pastoral system, 31 of the 284 households surveyed were affected by rabies during 2013-2014, resulting in a total of 73 affected cattle. |
|
The average herd-level incidence rate per village was 20.9% in the mixed crop-livestock system and 10.9% in the pastoral system; the overall herd-level incidence rate per village was 15.6%. The herd-level incidence rates per village were higher in the mixed crop-livestock system than in the pastoral system. The cattle-level incidence rates within a village were 2.2% in the mixed crop-livestock system and 1.7% in the pastoral system. The overall cattle-level incidence rate per village was 1.9%, while herd level incidence were 15.6%. |
|
Average economic losses per herd due to rabies in cattle were estimated at USD$49 per year for the mixed-crop livestock system, and at USD$52 per year for the pastoral system. However, in affected herds, average annual losses were as high as USD$228 (range USD$ 48–1016) in the mixed crop-livestock system and USD$477 (range USD$173–1140) in the pastoral system. The annual national economic loss due to rabies in cattle is estimated to be about USD$209 million in Ethiopia. |
To conclude, understanding the risk of rabies transmission and the socio-economic impact in Ethiopia appears to lay the foundation for initiating improved prevention and treatment strategies based on a One Health approach. In addition to extending knowledge of the clinical and economic impact of rabies in humans, dogs and cattle in Ethiopia, this field experience also identified equally important factors associated with this disease, including influences of the physical environment (agroecology, accessibility of health facilities), social environment (rural, urban), and human occupational environment (herders, language). Based on the findings of this field study, improvements in the rural districts with regard to accessibility of PET-delivering health centers at a shorter distance could significantly improve health-seeking behaviors.
References
- Beyene, T. J., Mourits, M. C., Kidane, A. H., & Hogeveen, H. (2018). Estimating the burden of rabies in Ethiopia by tracing dog bite victims. PloS One, 13(2), e0192313.
- Broban, A., Tejiokem, M. C., Tiembré, I., Druelles, S., & L’Azou, M. (2018). Bolstering human rabies surveillance in Africa is crucial to eliminating canine-mediated rabies. PLoS Negl Trop Dis, 12(9).
- Catley, A., Irungu, P., Simiyu, K., Dadye, J., Mwakio, W., Kiragu, J., & Nyamwaro, S. O. (2002). Participatory investigations of bovine trypanosomiasis in Tana River District, Kenya. Med Vet Entomol, 16(1), 55-66.
- Cleaveland, S., Fevre, E. M., Kaare, M., & Coleman, P. G. (2002). Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ, 80(4), 304-310.
- Debela BL. (2017). Factors affecting differences in livestock asset ownership between male- and female-headed households in northern Ethiopia. Eur J Dev Res, 29(2), 328-347.
- Hampson, K., Dobson, A., Kaare, M., Dushoff, J., Magoto, M., Sindoya, E., & Cleaveland, S. (2008). Rabies exposures, post-exposure prophylaxis, and deaths in a region of endemic canine rabies. PLoS Negl Trop Dis, 2(11).
- Jibat, T., Mourits, M. C., & Hogeveen, H. (2016). Incidence and economic impact of rabies in the cattle population of Ethiopia. Prev Vet Med, 130, 67-76.
- Kabeta, T., Deresa, B., Tigre, W., Ward, M. P., & Mor, S. M. (2015). Knowledge, attitudes and practices of animal bite victims attending an anti-rabies health center in Jimma Town, Ethiopia. PLoS Negl Trop Dis, 9(6).
- Kahn, C. M. & Line, S. (2010). The Merck Veterinary Manual, 10th ed. Whitehouse Station, NJ: Merck & Co., Inc.
- Okell, C. N., Pinchbeck, G. P., Stringer, A. P., Tefera, G., & Christley, R. M. (2013). A community-based participatory study investigating the epidemiology and effects of rabies to livestock owners in rural Ethiopia. Prev Vet Med, 108(1), 1-9.
- Teshome, E., Zenebe, M., Metaferia, H., & Biadgilign, S. (2014). Participation and significance of self-help groups for social development: exploring the community capacity in Ethiopia. SpringerPlus, 3(1), 189.
- Tepsumethanon, V., Wilde, H., & Meslin, F. X. (2005). Six criteria for rabies diagnosis in living dogs. J Med Assoc Thai, 88(3), 419-422.
Author
Center for Outcomes Research and Epidemiology, Kansas State University
College of Veterinary Medicine and Agriculture, Addis Ababa University
Next story: Giardia intestinalis in Argentina: A One Health Field Study
K-State Olathe
22201 W. Innovation Dr.
Olathe, KS 66061-1304
913-541-1220
913-541-1488 fax
olatheinfo@k-state.edu